iPS Cells (Induced Pluripotent Stem Cells)

Summary
Induced Pluripotent Stem Cells (iPS cells or iPSCs), was first introduced in 2006 by a Japanese stem cell researcher and a Nobel Prize laureate – Prof. YAMANAKA Shinya of Kyoto University, Japan. iPS cells hold immense potential due to their ability to differentiate into various cell types, mirroring embryonic stem cells’ characteristics. They also represent a groundbreaking technology with transformative implications for regenerative medicine. Their versatility, combined with their ability to bypass ethical controversies associated with embryonic stem cells, positions iPS cells at the forefront of biomedical research and therapeutic innovation. As researchers continue to unravel the intricacies of iPS cell biology and overcome existing challenges, the field holds the promise of delivering personalised, effective treatments for a wide range of debilitating diseases, ushering in a new era of regenerative medicine.
Understanding iPS Cells
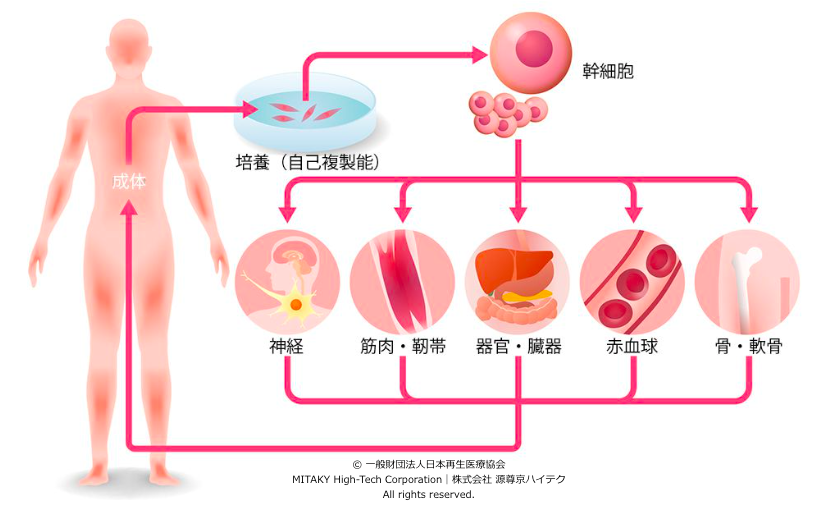
Induced Pluripotent Stem Cells (iPS cells) are reprogrammed adult cells, typically derived from fibroblasts or other somatic cells, through the introduction of specific transcription factors such as Oct3/4, Sox2, Klf4, and c-Myc. This reprogramming process resets the cellular epigenetic landscape, reverting the cells to a pluripotent state akin to embryonic stem cells (ESCs), without the ethical concerns associated with the use of embryos. iPS cells possess remarkable self-renewal capacity and the potential to differentiate into virtually any cell type in the human body, making them invaluable in regenerative medicine.
Five Key Characteristics of iPS Cells
- Pluripotency (Versatility): iPS cells possess the unique ability to differentiate into virtually any cell type in the human body, mirroring embryonic stem cells’ versatility.
- Reprogrammability: Through the introduction of specific transcription factors, iPS cells can be reprogrammed from adult somatic cells, offering a non-controversial alternative to embryonic stem cells.
- Disease Modeling: iPS cells enable the creation of disease-specific cell models, providing invaluable insights into disease mechanisms, drug discovery, and personalised medicine.
- Patient Specificity: By deriving iPS cells from patient samples, researchers can develop personalised therapies and drug screening platforms tailored to individual genetic profiles.
- Potential for Regenerative Medicine: iPS cells hold promise for cell replacement therapies, tissue regeneration, and transplantation, offering hope for treating a wide range of degenerative diseases and injuries.
Applications of iPS Cells in Regenerative Medicine:
1. Disease Modeling:
iPS cells offer a powerful platform for modeling human diseases in vitro, allowing researchers to recapitulate disease phenotypes and study underlying mechanisms. By reprogramming patient-derived cells into iPS cells and subsequently differentiating them into disease-relevant cell types, scientists can investigate disease progression, screen potential therapeutics, and personalize treatment approaches. For instance, researchers have utilized iPS cells to model neurodegenerative disorders like Parkinson’s and Alzheimer’s disease, cardiovascular diseases, and genetic disorders such as Duchenne muscular dystrophy.
2. Drug Discovery and Development:
The ability to generate patient-specific cell types from iPS cells facilitates personalised drug screening and development. iPS cell-derived models enable researchers to evaluate drug efficacy, toxicity, and side effects in a more physiologically relevant context, potentially reducing the attrition rates of drug candidates during clinical trials. Moreover, iPS cell-based platforms allow for the identification of novel therapeutic targets and the development of precision medicines tailored to individual patients, thereby advancing personalized healthcare.
3. Cell Replacement Therapy:
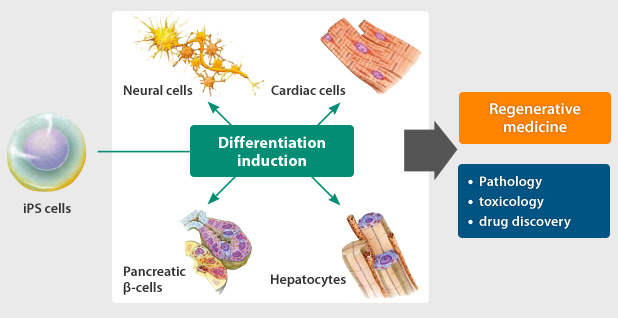
One of the most promising applications of iPS cells lies in cell replacement therapies for treating degenerative diseases and tissue injuries. Through directed differentiation protocols, iPS cells can be guided to differentiate into specific cell types required for tissue regeneration, such as cardiomyocytes for heart repair, dopaminergic neurons for Parkinson’s disease, and pancreatic β cells for diabetes. These differentiated cells can then be transplanted into patients to restore tissue function, offering potential cures for previously incurable conditions.
Challenges
While iPS cells hold immense promise, several challenges need to be addressed to fully realise their potential in regenerative medicine. These include concerns regarding the safety and efficacy of iPS cell-based therapies, issues related to the scalability and standardisation of cell manufacturing processes, and ethical considerations surrounding the use of genetic manipulation techniques. Additionally, advancements in gene editing technologies such as CRISPR-Cas9 hold promise for enhancing the precision and efficiency of iPS cell reprogramming and differentiation.
A journey to stem cell research of Prof. Yamanaka
Professor Yamanaka Shinya’s began his journey with a passion for medicine and a desire to alleviate human suffering. After earning his medical degree from Kobe University in Japan in 1987, Yamanaka embarked on a career in orthopedic surgery. However, he soon realised that surgery alone could not address the root causes of many diseases.
Driven by a desire to delve deeper into the mechanisms of disease and develop more effective treatments, Yamanaka decided to pursue a career in basic research. He joined the Gladstone Institute of Cardiovascular Disease at the University of California, San Francisco, where he began studying the genetic factors underlying heart disease.
Inspired by the potential of embryonic stem cells to revolutionise regenerative medicine, Dr. Yamanaka shifted his focus to stem cell research. He became intrigued by the idea of reprogramming adult cells into a pluripotent state, thereby bypassing the ethical concerns associated with embryonic stem cells.
In 2004, Dr. Yamanaka returned to Japan and joined Kyoto University as a professor at the Center for iPS Cell Research and Application (CiRA). At Kyoto University, he had the resources and support to pursue his groundbreaking research on induced pluripotent stem cells (iPS cells).
Through tireless experimentation and innovative thinking, Prof. Yamanaka and his team succeeded in reprogramming adult cells into iPS cells in 2006. This achievement not only established Yamanaka Shinya as a leading figure in stem cell research but also positioned Kyoto University as a global hub for regenerative medicine.
Prof. Yamanaka’s groundbreaking work at Kyoto University earned him numerous accolades, including the Nobel Prize in Physiology or Medicine in 2012. Today, he continues to lead pioneering research at CiRA, seeking to harness the potential of iPS cells to develop innovative therapies for a wide range of diseases and injuries.
Powered by MITAKY High-Tech Corporation|株式会社 源尊京ハイテク
References
- Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663-676.
- Yu, J., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318(5858), 1917-1920.
- Trounson, A., & DeWitt, N. D. (2016). Pluripotent stem cells progressing to the clinic. Nature Reviews Molecular Cell Biology, 17(3), 194-200.
- Saha, K., & Jaenisch, R. (2009). Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell, 5(6), 584-595.
- Mandai, M., et al. (2017). Autologous induced stem-cell-derived retinal cells for macular degeneration. New England Journal of Medicine, 376(11), 1038-1046.
- Cyranoski, D. (2018). Japan approves first human-animal embryo experiments. Nature, 564(7734), 16-17.