Regenerative Medicine in Japan
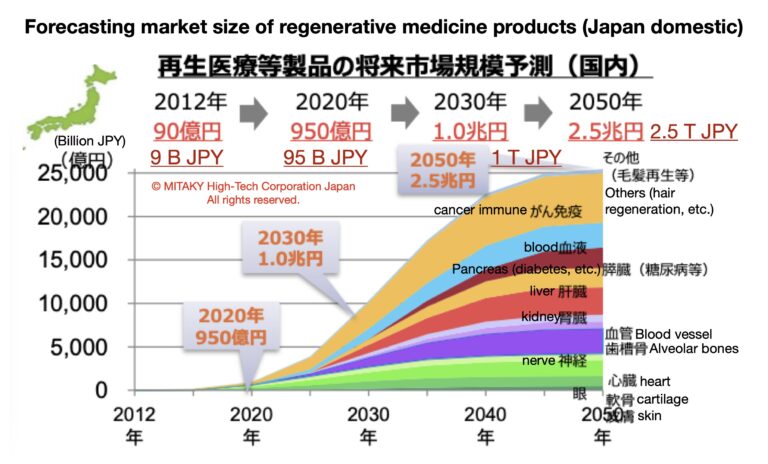
Regenerative Medicine Products Market Size in Japan and Worldwide until 2050
In Japan, the regenerative medicine market is poised for significant expansion, propelled by supportive government policies, technological innovations, and a strong ecosystem of research institutions and biotechnology companies. The market size in Japan was estimated to be around $2.5 billion in 2022, with projections indicating a CAGR of approximately 30% from 2022 to 2027, reaching a value of over $10 billion by 2027.
As of the world, the global regenerative medicine market was valued at around $24 billion and is expected to grow at a compound annual growth rate (CAGR) of approximately 23% from 2022 to 2027, reaching a value of over $90 billion by 2027. This growth trajectory is fueled by the commercialisation of innovative products across various therapeutic areas, including orthopedics, cardiovascular diseases, and oncology.

Looking further ahead to 2050, the regenerative medicine market is expected to continue its upward trajectory, driven by advancements in stem cell biology, tissue engineering, and gene editing technologies. While precise forecasts beyond 2027 are speculative, analysts anticipate sustained growth fueled by the increasing demand for regenerative therapies to address aging populations, chronic diseases, and unmet medical needs.
By 2050, the global regenerative medicine market could potentially exceed $500 billion, with Japan continuing to play a significant role as a key innovator and market leader in this transformative field. Continued investment in research, regulatory harmonisation, and healthcare infrastructure will be essential to realise the full potential of regenerative medicine and improve patient outcomes worldwide.
Regenerative Medicine: A Paradigm Shift
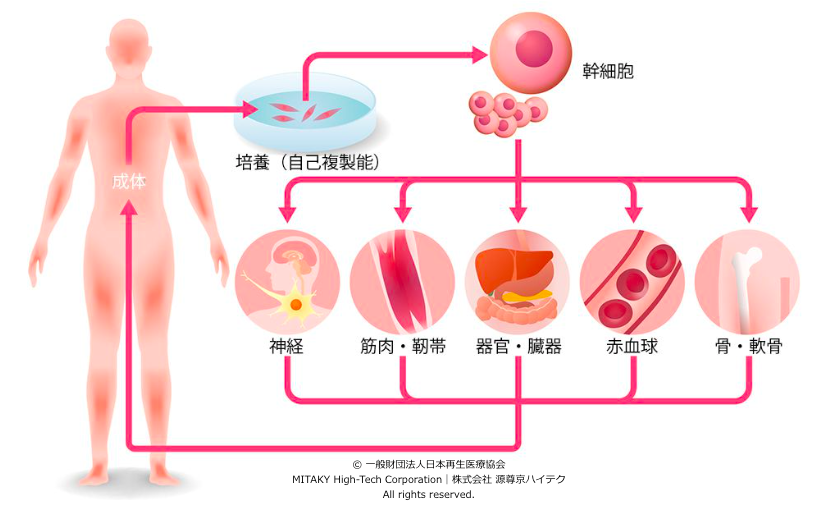
Regenerative medicine represents a paradigm shift in healthcare, aiming to restore the structure and function of damaged tissues and organs through the stimulation of the body’s own repair mechanisms or by introducing exogenous cells, tissues, or biomaterials. Stem cell therapy, tissue engineering, and gene therapy are key pillars of regenerative medicine, offering promising avenues for treating a wide range of conditions, from cardiovascular diseases to neurodegenerative disorders.
Japan's Commitment to Regenerative Medicine
Japan has emerged as a global hub for regenerative medicine, driven by a combination of scientific expertise, government support, and regulatory reforms. In 2014, the Japanese government implemented the Regenerative Medicine Promotion Act, streamlining the approval process for regenerative medicine products and establishing a framework for their clinical development and commercialization. This legislation marked a significant milestone, facilitating collaboration between academia, industry, and regulatory agencies to accelerate the translation of scientific discoveries into clinical applications.
Breakthrough Products and Innovations in Japan
Several breakthroughs in regenerative medicine have originated from Japan, demonstrating the country’s leadership in this field. One notable example is induced pluripotent stem cells (iPSCs), pioneered by Dr. Shinya Yamanaka of Kyoto University. iPSCs are reprogrammed adult cells capable of differentiating into various cell types, offering a potentially limitless source of patient-specific cells for transplantation and drug discovery. The discovery of iPSCs earned Dr. Yamanaka the Nobel Prize in Physiology or Medicine in 2012 and sparked a surge of research in regenerative medicine worldwide.
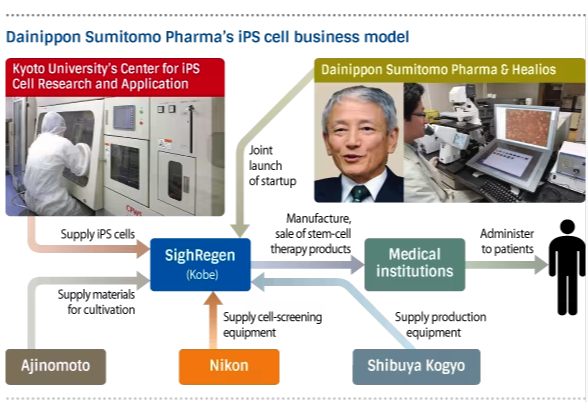
Building upon this foundation, Japanese companies and research institutions have developed a diverse array of regenerative medicine products targeting different therapeutic areas. For instance, Healios, a biotechnology company based in Tokyo, is conducting clinical trials of regenerative cell therapy for ischemic stroke, a leading cause of disability worldwide. By transplanting neural stem cells derived from human iPSCs into the brains of stroke patients, Healios aims to promote tissue repair and functional recovery, potentially revolutionising the treatment of this devastating condition.
In the field of tissue engineering, Organ Technologies is pioneering hair regeneration therapies for the treatment of baldness. Leveraging advances in cell culture techniques and 3D bio-printing, the company aims to engineer hair follicles from patients’ own cells and implant them into the scalp to stimulate hair growth. This innovative approach offers a promising solution for millions of individuals suffering from alopecia, addressing a significant unmet medical need.
Furthermore, Japan has made significant strides in the development of gene therapies, particularly for genetic disorders and rare diseases. An illustrative example is the work of Dr. Fumihiro Sugiyama and his team at Osaka University, who are investigating gene editing technologies such as CRISPR-Cas9 to correct genetic mutations underlying inherited retinal diseases. By precisely targeting and repairing faulty genes in retinal cells, these therapies hold the potential to restore vision and improve the quality of life for patients with debilitating eye conditions.
Regulatory Framework
While Japan has made commendable progress in advancing regenerative medicine, the field faces several regulatory and logistical challenges. Despite the streamlined approval process introduced by the Regenerative Medicine Promotion Act, ensuring the safety and efficacy of novel therapies remains paramount. Regulatory agencies such as the Pharmaceuticals and Medical Devices Agency (PMDA) play a critical role in evaluating and monitoring regenerative medicine products, balancing the need for innovation with rigorous oversight to protect patient safety.
Challenges
Moreover, the scalability and cost-effectiveness of regenerative medicine products pose practical challenges for widespread adoption. Manufacturing complex cell-based therapies at a commercial scale requires sophisticated infrastructure and quality control measures, which can drive up production costs and limit accessibility for patients. Addressing these challenges will require continued investment in research and development, as well as collaboration between academia, industry, and regulatory bodies to optimize manufacturing processes and streamline supply chains.
Future and Opportunities
Looking ahead, the future of regenerative medicine in Japan appears promising, with ongoing research efforts poised to yield further breakthroughs and therapeutic innovations. Advances in stem cell biology, tissue engineering, and gene editing technologies continue to expand the possibilities for regenerative therapies, offering hope for patients with degenerative diseases and chronic conditions.
Furthermore, collaborations between academia, industry, and government are essential for translating scientific discoveries into tangible medical solutions and driving the growth of Japan’s regenerative medicine sector. Initiatives such as the Japan Agency for Medical Research and Development (AMED) provide critical funding and infrastructure support for regenerative medicine research, fostering a vibrant ecosystem of innovation and entrepreneurship.
Powered by MITAKY High-Tech Corporation|株式会社 源尊京ハイテク
References
- Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663-676.
- Cyranoski, D. (2014). Japanese woman is first recipient of next-generation stem cells. Nature, 513(7519), 287.
- Matsui, H., & Watanabe, E. (2020). Regenerative medicine in Japan today: A summary of the legislation and achievements. Cell Stem Cell, 27(3), 404-408.
- Sugiyama, F., & Ikeda, Y. (2019). CRISPR-Cas9-based genome editing for the development of next-generation CAR-T cell therapy against solid tumors. Cancer Science, 110(10), 2683-2689.
- Organ Technologies Inc. (2024). Hair Regeneration.
- Healios K.K. (2024). Clinical Trials.
- Yamasaki, D., Arai S. (2014). Nikkei Asia – Japan’s stem-cell therapy progress accelerates