Japanese Regenerative Medicine Technology Transfer
Japanese renowned regenerative medical technologies include Stem cell therapy for many treatments and Immune cell therapy for cancer treatment
Especially our stem cell therapy helps leverage the body’s natural healing abilities, aiming to repair damaged tissues and organs. With the potential to treat a wide range of conditions from injuries to degenerative diseases, it represents a groundbreaking approach towards restoring health and improving quality of life.
What is Technology Transfer?
Technology transfer is the process where an organisation possessing an advanced technology provides it to a developing organisation, facilitating the exchange of know-how, knowledge, skills, and innovations to enhance their capabilities.

Benefits of Technology Transfer to Businesses
Cost and Time Efficiency
Technology transfer saves your company from investing in extensive R&D, providing ready-to-use solutions that fit your production needs, from raw materials to manufacturing processes, reducing both time and financial outlay.
Risk Reduction
By adopting proven technologies from our successful companies in Japan, your businesses can avoid the costly and time-consuming process of trial and error, significantly minimising risks in your manufacturing processes.
Access to Cutting-Edge Technology
Your businesses could benefit from the latest, most efficient technologies of our Japanese partners through technology transfer, leading to enhanced your productivity and finally significant economic advantages.
READy-to-transfer Technologies
Our Japanese partners hold exceptional research findings, know-how, and patents in stem cell and immune cell cultivation and storage. We have successfully applied stem cell therapies for various conditions as well as immune cell therapy for cancer treatment and prevention in clinical settings across Japan. We are confident in our ability to share and transfer these technologies to partners globally, either in full or in part, including setting up GMP laboratories, cell culturing know-how, quality controlling, patient counselling, and ultimately applying the therapies in patient treatment. Both technologies have approved by our Japanese Ministry of Health, Labour and Welfare according to the “Act on the Safety of Regenerative Medicine” (ASRM) and the “Pharmaceuticals and Medical Devices Act” (PMD Act).
Stem cell cultivation technology
for clinical use
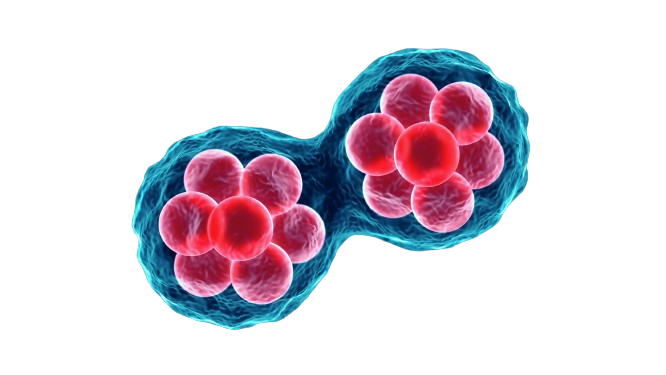
Adipose tissue-derived stem cell (ADSCs) technology uses stem cells from fat tissue of own patient for treatments like tissue repair, anti-aging, stroke, dementia, arteriosclerosis, knee pain and managing degenerative diseases. It’s a promising method that’s minimally invasive.
IMMUNE cell cultivation technology
for clinical use
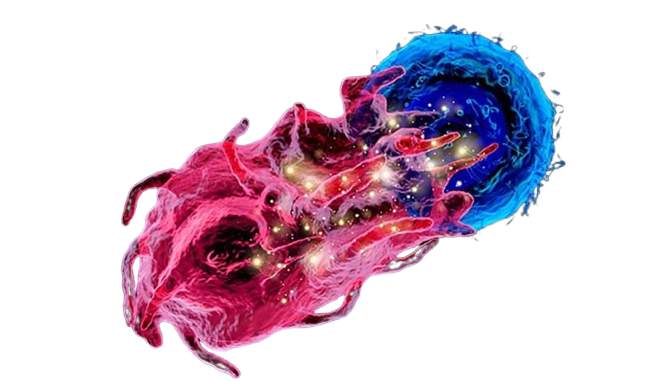
Autologous immune cell technology uses a patient’s own immune cells to fight diseases like cancer (lung, liver, breast, pancreatic, and many other cancers). By enhancing and reintroducing these cells, it offers personalised and targeted treatment with fewer side effects.
Japanese Excellence in Stem Cell Research
Japan has been at the forefront of stem cell research, with significant contributions from world-renowned scientists. Notably, Dr. Shinya Yamanaka was awarded the Nobel Prize in Physiology or Medicine in 2012 for his groundbreaking discovery of induced pluripotent stem cells (iPSCs). His work demonstrated that mature cells can be reprogrammed to become pluripotent, offering immense potential in regenerative medicine, including applications in ADSC therapy.
The contributions of Dr. Yamanaka and other Japanese researchers have positioned Japan as a leader in this regenerative medicines field, offering patients access to some of the most advanced stem cell therapies in the world. MITAKY Group’s network of partner institutions continues this legacy, integrating the latest scientific advancements into their treatment protocols.
Process
At MITAKY, we offer comprehensive technology transfer support services for your company’s development of novel regenerative medical technologies, encompassing development, production, high-end equipment logistics, patient consultation, and clinical applications.
Consultation・Kickoff Meeting
✉️ Send your request to email: business@mitaky.co.jp
☎️ Hotline: +81-50-6875-7799
Planning & Proposal
We will carefully consider your requests and propose strategic partners, laboratory design under GMP conditions, culturing machine and equipment specifications, and others. Additionally, we will offer expert advice on creating an attractive product within your budget.
Technical Expert Meeting
During the proposal phase, we will hold a crucial ‘Technical Expert Meeting’ between your team and our Japanese experts & researchers in regenerative medical technologies. The purpose of this meeting is to clarify your technical objectives and ensure that we provide the right technologies for your project.
Quotation
Once you are satisfied with our proposal, we will provide you with a detailed quote. This will include cost estimates for each step of the development process, as well as several package options. This way, you can choose the final package that best aligns with your needs and preferences.
Contract Conclusion
The official contract signing will mark the formal commencement of the project between your company and our Japanese partners and associates.
Project Start
The project is official started, where all key stakeholders, including your team and our Japanese partners, will come together to align on the project goals, timelines, and deliverables of either stem cell or immune cell culturing technologies implementing at your countries/organisations.
Ready to Start Your PROJECT?
✉️ Send your request to email: business@mitaky.co.jp
☎️ Hotline: +81-50-6875-7799 (Tamura & Takahashi)



