Japanese Autologous Adipose Tissue-Derived Stem Cell Therapy
MITAKY Group is a premier facilitator of high-quality medical and healthcare tourism support services in Japan. We specialise in connecting international patients with Japan’s most advanced medical centres, offering access to cutting-edge treatments such as Autologous Adipose Tissue-Derived Stem Cell (ADSC) Therapy to treat Stroke, Anti ageing, Arteriosclerosis, Dementia, knee pain, and others. We prides ourselves on our extensive network of accredited institutions, ensuring that our clients receive the highest standard of care available in Japan.
- What is Regenerative Medicine? ↓
- What is Japanese Autologous Adipose Tissue-Derived Stem Cell (ADSC) Therapy? ↓
- Scientific Basis and Effectiveness of ADSC Therapy ↓
- Five Conditions Can Be Treated with ADSC Therapy in MITAKY ↓
- Process of ADSC Therapy ↓
- Why Choose MITAKY Group for Your Stem Cell Therapy? ↓
- Japanese Excellence in Stem Cell Research ↓
- Treatment Costs ↓
- Our Medical Partners’ Facilities ↓
- FAQs ↓
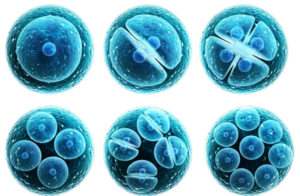
What is Regenerative Medicine?
Regenerative medicine is an innovative and rapidly evolving field in medical science that focuses on repairing, replacing, and regenerating damaged tissues and organs. It harnesses the body’s natural healing processes by using cutting-edge technologies such as immune cell therapy and stem cell therapy.
Key approaches include:
- Immune Cell Therapy: Immune cells, such as T-cells and Natural Killer (NK) cells, are extracted from the patient’s own blood. These cells are then activated, expanded, and reinfused into the body to target and destroy cancer cells, offering a personalised and precise treatment option.
- Stem Cell Therapy: Stem cells are typically extracted from adipose (fat) tissue or bone marrow. These versatile cells can differentiate into various cell types to repair damaged tissues, regenerate cells, and address degenerative diseases or injuries.
These therapies exemplify the potential of regenerative medicine to address the root causes of diseases and restore health.
What is Japanese Autologous Adipose Tissue-Derived Stem Cell Therapy?
Japanese Autologous Adipose Tissue-Derived Stem Cell (ADSC) Therapy is an advanced form of regenerative medicine facilitated through MITAKY Group’s network of esteemed medical centres in Japan. This therapy involves collecting stem cells from a patient’s own adipose (fat) tissue, processing them, and reinfusing them into the patient’s body to promote healing and regeneration of damaged tissues. The use of the patient’s own cells minimises the risk of rejection and eliminates ethical concerns, ensuring a safe and personalised treatment experience.
This therapy is approved by the Ministry of Health, Labour and Welfare of Japan under the “Act on the Safety of Regenerative Medicine” (ASRM, Law No. 85 of 2013), ensuring that it meets the highest standards of safety and efficacy.
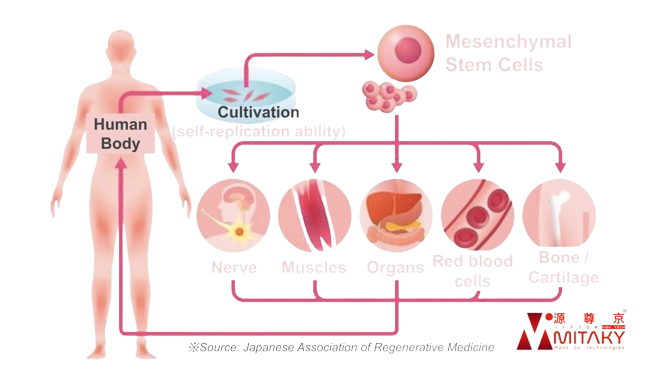
Scientific Basis and Effectiveness of ADSC Therapy
ADSC Therapy provided through MITAKY Group’s partner institutions is grounded in robust scientific research and clinical practice. ADSCs are capable of differentiating into various cell types, including fat, bone, and cartilage cells. Additionally, these cells exhibit potent anti-inflammatory and immunomodulatory properties, making them highly effective in treating a range of conditions. Research and clinical data have demonstrated their effectiveness in conditions such as stroke, dementia, and arteriosclerosis
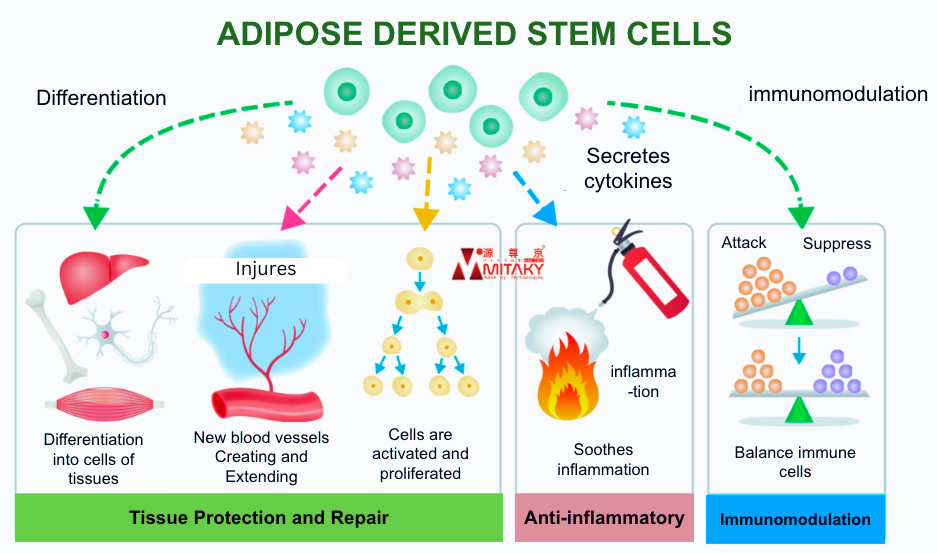
A unique and crucial feature of ADSCs is their homing ability. This means that when ADSCs are introduced into the body, they possess an innate ability to migrate or “home” to sites of injury, inflammation, or disease. Once they arrive at these sites, they initiate repair processes by differentiating into the needed cell types and releasing cytokines and growth factors that aid in tissue regeneration and immune modulation.
Homing Mechanism: ADSCs express a variety of receptors on their surfaces that detect signals from injured or inflamed tissues. These signals are typically chemokines or other molecules that guide the ADSCs to the specific location in the body where they are needed most. Once at the injury site, the ADSCs exert their therapeutic effects by:
- Promoting angiogenesis (formation of new blood vessels): This is critical in restoring blood supply to damaged tissues.
- Modulating the immune response: They help reduce chronic inflammation, which is often a contributor to tissue damage.
- Facilitating tissue repair: By differentiating into the required cell types, such as cartilage cells in joints or neurons in the brain, ADSCs directly contribute to the repair and regeneration of tissues.
Five Conditions Can Be Treated with ADSC Therapy in MITAKY
Patients connected through MITAKY Group can access ADSC therapy in Japan for the following conditions: stroke, arteriosclerosis, dementia, knee pain, anti ageing and many other symptoms.
STROKE
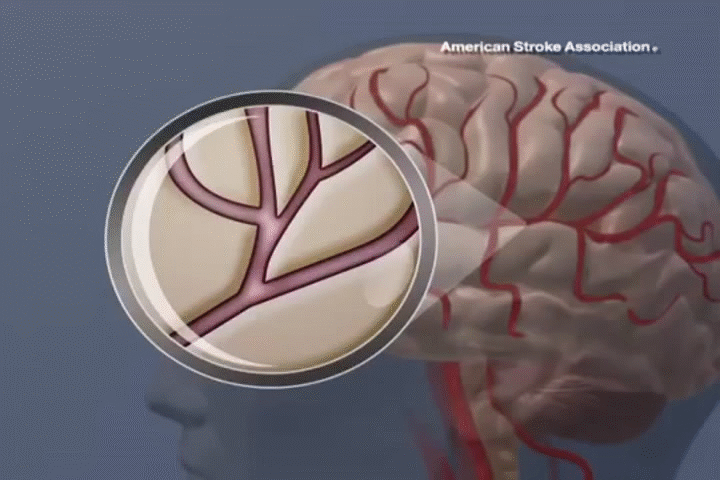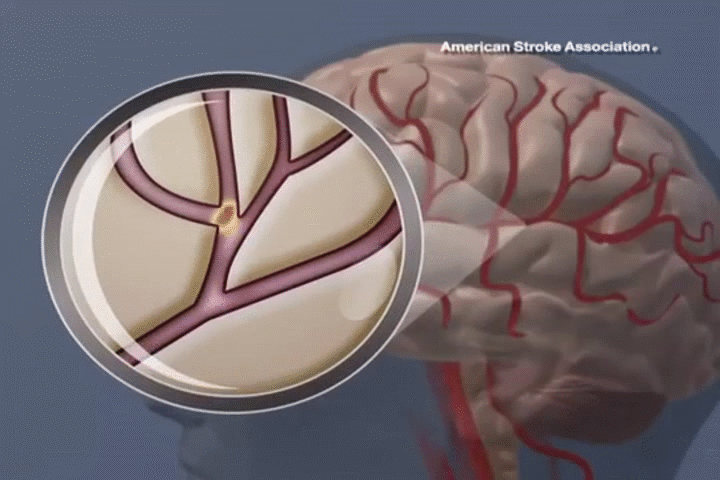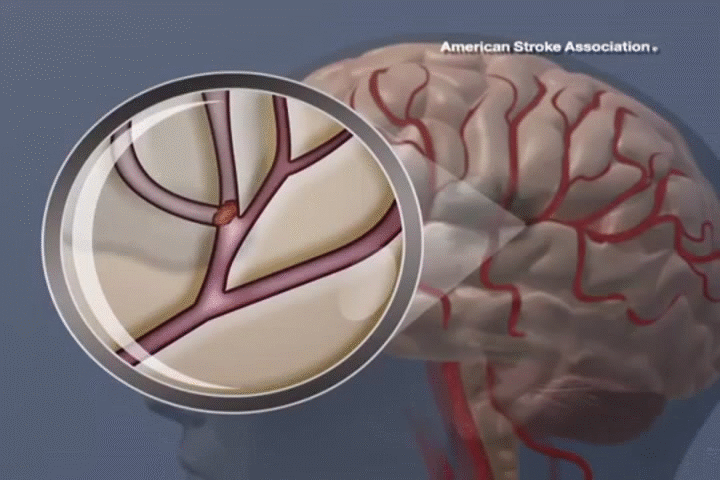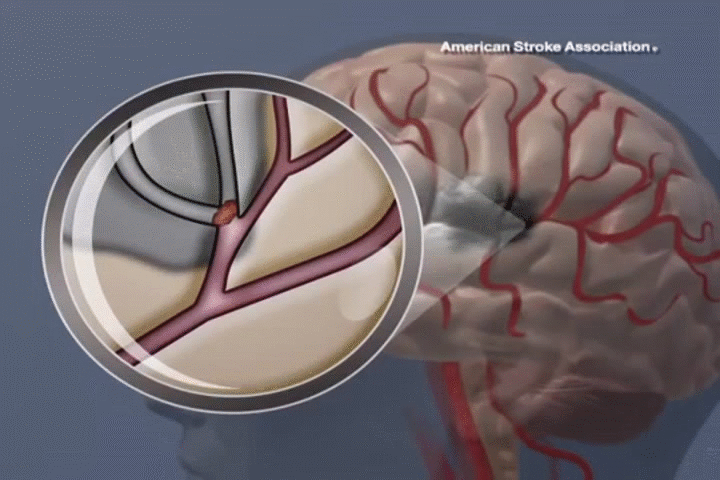
ADSC therapy promotes neural regeneration and reduces inflammation, resulting in significant improvements in mobility and cognitive function
Arteriosclerosis
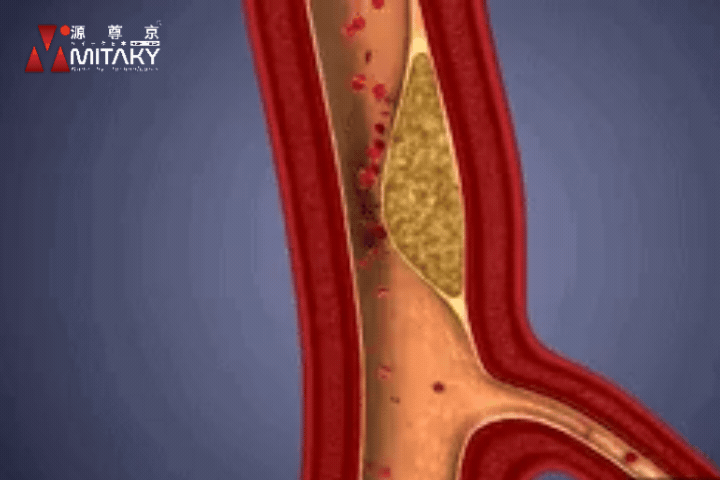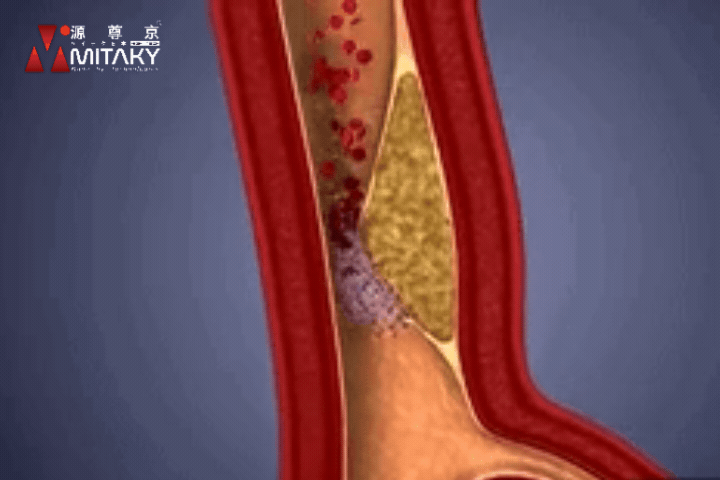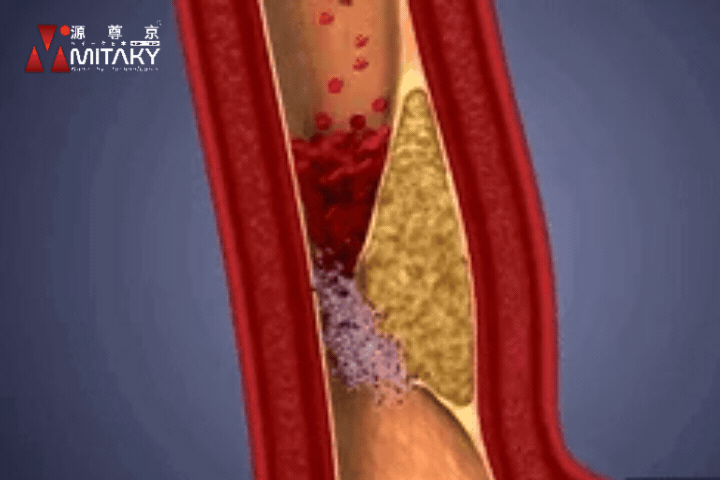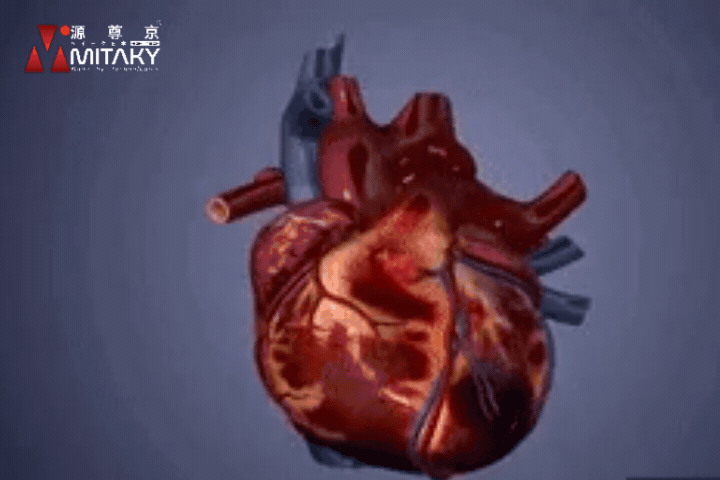
ADSC therapy reduces arterial stiffness by up to 30%, significantly improving vascular health and reducing the risk of cardiovascular events
Dementia
The therapy has been shown to slow cognitive decline and enhance brain function, improving patients’ quality of life
Knee Pain
Patients with osteoarthritis have experienced up to a 50% reduction in pain and marked improvements in joint function following ADSC therapy
Anti-Ageing

ADSC therapy is also used in aesthetic medicine, enhancing skin elasticity by 35% and reducing wrinkles by 25%, contributing to a more youthful appearance
Process of ADSC Therapy
MITAKY Group ensures a seamless experience for international patients seeking ADSC therapy through our partner medical centers. The treatment process includes:
Consultation
✉️ Send your request to email: info@mitaky.co.jp
☎️ Hotline: +81-50-6875-7799
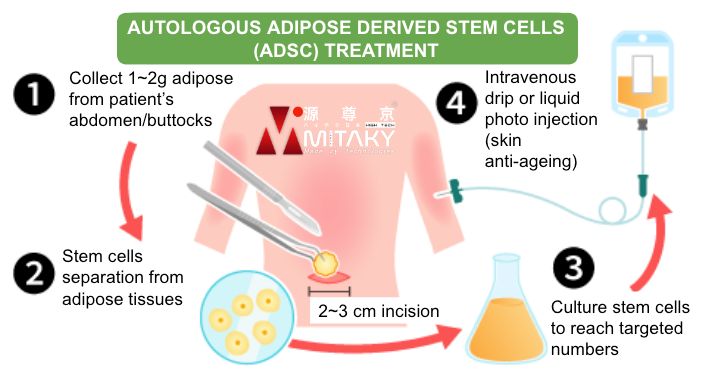
Adipose Collection
After the treatment plan is confirmed and all essential tests have been conducted, approximately 50-100 mL (1-2 gram) of adipose tissue is extracted from the abdominal area or buttocks of the patient. From a single extraction, stem cells can be cultured for approximately 6 treatment sessions. The processed cell products can be stored for a maximum of 2 years.
Cell Cultivation & Processing
Extracted tissue is processed, cultured, and expanded in certified Cell Processing Centres (CPCs) that adhere to Good Manufacturing Practice (GMP) standards, ensuring the highest quality and safety of the stem cells.
This process is fully compliant with the Japan’s Ministry of Health, Labour and Welfare’s “Act on the Safety of Regenerative Medicine” (ASRM) and the “Pharmaceuticals and Medical Devices Act” (PMD Act), ensuring that all procedures meet Japan’s stringent safety and regulatory standards.
What is CPC?
A Cell Processing Centre (CPC, 細胞培養加工施設) is a dedicated clean room that maintains the necessary cleanliness required for culturing cells. It is a specialised facility designed for handling and processing biological materials, such as stem cells, under stringent conditions. These facilities comply with the Ministry of Health, Labour and Welfare’s “Act on the Safety of Regenerative Medicine” (ASRM) and the “Pharmaceuticals and Medical Devices Act” (PMD Act). Additionally, to operate a CPC, the facility must obtain a specific facility number issued by the Minister of Health, Labour and Welfare in accordance with the ASRM. This ensures that all procedures meet Japan’s stringent safety and regulatory standards. CPCs operate under Good Manufacturing Practice (GMP) guidelines, which are internationally recognised standards in medical therapy production. Our partner centres follow these strict protocols to prevent contamination and ensure that the cells are of the highest quality.
Reinfusion
Processed stem cells are reinfused into the patient’s body, targeting areas in need of regeneration. This outpatient procedure is minimally invasive and typically completed within a few hours.
Monitoring & Follow-Up
Regular monitoring and evaluations are conducted at 1, 3, 6, and 12 months post-infusion to assess treatment effectiveness and make any necessary adjustments.
Why Choose MITAKY Group for Your Stem Cell Therapy?
Access to Advanced
Technology
Our Japanese partner medical centres & institutes utilise the latest cell processing techniques, ensuring highly effective treatments with over 95% cell viability. Using own cells to maximise safety and effectiveness.
Absolute
Confidentiality
MITAKY Group is deeply committed to protecting the privacy and confidentiality of our patients, especially those who are affluent or high-profile individuals from businessmen and businesswomen to celebrities.
Personalised
Care
Treatments are tailored to each patient’s unique needs. With comprehensive supports in Japan: visa assistance, transportation, medical documents translation, professional interpretation, luxury shopping, resorts and golf clubs information.
Scientific
Validation
Our methods are supported by extensive research and clinical trials, ensuring scientifically validated treatments for conditions such as stroke, dementia, arteriosclerosis, knee pain, anti ageing and others.
Global
Standards
Our protocols meet international guidelines, offering treatments that are both safe and effective, recognised by health authorities worldwide.
Certified
Facilities
All stem cell processing and cultivation occur in CPC facilities that adhere to GMP standards, ensuring the highest quality and safety.
Japanese Excellence in Stem Cell Research
Japan has been at the forefront of stem cell research, with significant contributions from world-renowned scientists. Notably, Dr. Shinya Yamanaka was awarded the Nobel Prize in Physiology or Medicine in 2012 for his groundbreaking discovery of induced pluripotent stem cells (iPSCs). His work demonstrated that mature cells can be reprogrammed to become pluripotent, offering immense potential in regenerative medicine, including applications in ADSC therapy.
The contributions of Dr. Yamanaka and other Japanese researchers have positioned Japan as a leader in the field, offering patients access to some of the most advanced stem cell therapies in the world. MITAKY Group’s network of partner institutions continues this legacy, integrating the latest scientific advancements into their treatment protocols.
Treatment Costs
The cost of ADSC therapy varies based on individual patient needs and treatment complexity. Through MITAKY Group, patients can access competitive pricing from our partner medical centres & institutes:
- Initial Consultation and Testing: Starting from ¥110,000 (~USD 733).
- Basic Packages: Starting at JPY 2,400,000 (approximately USD 16,300).
- Comprehensive Packages: Including multiple infusions and extended follow-up care, ranging from JPY 2,900,000 to JPY 5,500,000 (approximately USD 19,000 to USD 37,000)
- Additional Fees: May apply for specialised tests or procedures.
Reference Exchange Rate: 1 JPY = 0.0068 USD.
Note: Prices include a 10% consumption tax. Final costs depend on individual treatment plans and specific medical needs. For more details, please contact MITAKY Group directly.
Our MEDICAL PARTNERS FACILITIES
The stem cells are prepared at our own certified on-site cell culture and processing centre (CPC) for the therapy.
We also store the cells within our reliable management system that complies with national and legal standards, specially to our Japan’s Ministry of Health, Labor and Welfare.





Frequently Asked Questions
Yes, ADSC therapy is safe as it uses autologous cells (your own), minimising the risk of immune rejection and adverse reactions. All procedures are conducted under stringent medical guidelines in CPC-certified facilities by Japan’s Ministry of Health, Labour & Welfare, to ensure the highest standards of safety and quality.
The entire process, from initial consultation to reinfusion, typically takes 4-6 weeks. The reinfusion itself is a one-day outpatient procedure with several hours only.
Results vary depending on the condition treated, but most patients report noticeable improvements within 1-3 months post-infusion.
The procedure is minimally invasive, with most patients experiencing only mild discomfort during the extraction and reinfusion phases.
The longevity of the treatment’s effects depends on the individual and the condition treated. However, many patients experience long-term benefits, with some reporting improvements lasting several years.
Before the therapy, patients should avoid smoking, excessive alcohol consumption, and strenuous activities. After the procedure, it’s recommended to avoid heavy exercise and stress on the treated area for a few days. Our Japanese doctors & physicians will provide specific guidelines based on your individual treatment plan.
ADSC therapy is generally safe for patients with various medical conditions, but it is crucial to have a thorough consultation with our Japanese physician & doctors. Conditions such as uncontrolled diabetes, severe infections, or active cancers may require special consideration before proceeding with therapy.
Like any medical procedure, ADSC therapy carries some risks, though they are minimal. Potential risks include infection at the injection site, temporary pain, and swelling. However, serious complications are very rare due to the autologous nature of the cells used.
Yes, ADSC therapy can often be combined with other treatments, such as physical therapy, medications, or even other regenerative medicine techniques, to enhance overall outcomes. Our Japanese physician & doctor will advise on the best combination of treatments for your specific condition.
The number of sessions required varies depending on the condition being treated and the patient’s response to the therapy. Some patients may achieve significant improvement after a single session, while others may benefit from multiple sessions spaced out over several months.
Yes, follow-up care is essential to monitor the effectiveness of the treatment and to ensure that there are no complications. Follow-up visits are typically scheduled at 1, 3, 6, and 12 months post-treatment.
MITAKY Group offers comprehensive support for international patients, including assistance with travel arrangements, accommodation, and interpretation services. We ensure that all aspects of your treatment and stay in Japan are managed smoothly.
Coverage for ADSC therapy varies depending on your insurance provider and policy. Since this therapy is considered advanced and sometimes experimental, it may not be covered by all insurance plans. We recommend checking with your insurance provider and discussing financing options with our team.
MITAKY Group is committed to providing international patients with access to Japan’s highest quality medical treatments through our network of accredited medical centres & institutes. By choosing MITAKY, you benefit from world-class care, cutting-edge technology, and personalised treatment plans that leverage the latest advancements in regenerative medicine. Let us connect you to a healthier, brighter future.
For more information or to schedule a consultation, please contact our support team by an official enquiry form below or by:
- Email at info@mitaky.co.jp (Takahashi, and Tamura)
- Hotline: +81-50-6875-7799



