Japanese Autologous Immune Cell Therapy for Cancer (Immunotherapy)
MITAKY Group is a premier facilitator of high-quality medical and healthcare tourism support services in Japan. We specialise in connecting international patients with Japan’s most advanced medical centres, offering access to cutting-edge cancer treatments and cancer preventions such as Japanese Autologous Immune Cell Therapy (Immunotherapy). We prides ourselves on our extensive network of accredited institutions, ensuring that our clients receive the highest standard of care available in Japan.
- What is Regenerative Medicine? ↓
- What is Japanese Autologous Immune Cell Therapy? ↓
- Scientific Basis and Effectiveness of Immune Cell Therapy ↓
- Types of Cancer Treated with Our Immune Cell Therapy ↓
- Process of Immune Cell Therapy ↓
- Why Choose MITAKY Group for Your Immune Cell Therapy? ↓
- Japanese Excellence in Immune Cell Research ↓
- Treatment Costs ↓
- Our Medical Partners’ Facilities ↓
- FAQs ↓
What is Regenerative Medicine?
Regenerative medicine is an innovative and rapidly evolving field in medical science that focuses on repairing, replacing, and regenerating damaged tissues and organs. It harnesses the body’s natural healing processes by using cutting-edge technologies such as immune cell therapy and stem cell therapy.
Key approaches include:
- Immune Cell Therapy: Immune cells, such as T-cells and Natural Killer (NK) cells, are extracted from the patient’s own blood. These cells are then activated, expanded, and reinfused into the body to target and destroy cancer cells, offering a personalised and precise treatment option.
- Stem Cell Therapy: Stem cells are typically extracted from adipose (fat) tissue or bone marrow. These versatile cells can differentiate into various cell types to repair damaged tissues, regenerate cells, and address degenerative diseases or injuries.
These therapies exemplify the potential of regenerative medicine to address the root causes of diseases and restore health.
What is Japanese Autologous Immune Cell Therapy?
Japanese Autologous Immune Cell Therapy or Cancer Immunotherapy is a personalised cancer treatment that utilises the patient’s own immune cells to combat cancer. This therapy involves extracting immune cells, such as T-cells and Natural Killer (NK) cells, from the patient, activating and expanding them in a controlled environment, and then reinfusing them into the patient’s body. The goal is to enhance the immune system’s ability to recognise and destroy cancer cells.
This approach is considered a fourth modality of cancer treatment, complementing surgery, radiotherapy, and chemotherapy. It offers a targeted method to eliminate cancer cells while minimising damage to healthy tissues. By using the patient’s own cells, the therapy reduces the risk of immune rejection and adverse reactions, providing a personalised and effective treatment option.
Scientific Basis and Effectiveness of Immune Cell Therapy
The Relationship Between Cancer and Immunity
In response to external influences such as living habits, age, and environment, reactions in the human body are disturbed, thereby producing abnormal cells (i.e. cancer cells). However, our bodies are also innately equipped with the ability to control the proliferation of cancer cells and prevent the development of disease, which is Immunity. When our immunity weakens (due to reasons such as living habits, eating habits, age, etc.), it becomes one of the causes of cancer development.
The immune system plays a crucial role in identifying and eliminating abnormal cells, including cancer cells, through a process known as immune surveillance. However, cancer cells can develop mechanisms to evade immune detection, such as:
- Immune Checkpoint Manipulation: Exploiting pathways like PD-1/PD-L1 to suppress T-cell activity.
- Immunosuppressive Microenvironment: Creating an environment that inhibits immune cell function.
- Reduced Antigen Presentation: Downregulating or masking antigens to avoid detection.
These evasion tactics allow cancer cells to proliferate and spread, making treatment challenging.
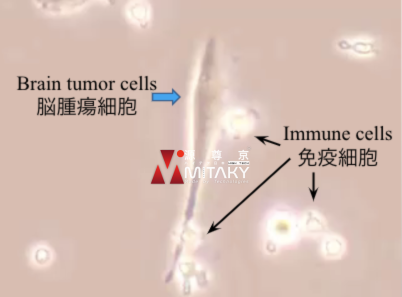
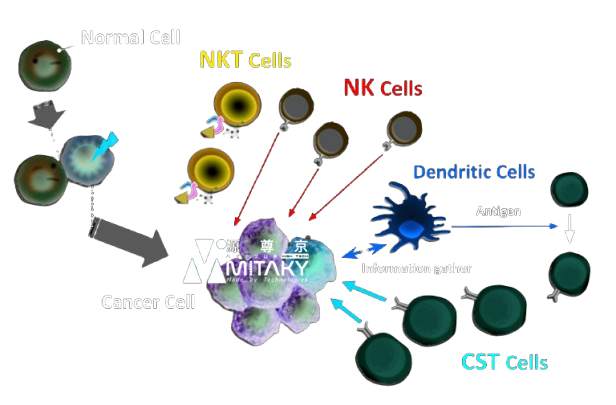
How Autologous Immune Cell Therapy Works
Autologous Immune Cell Therapy enhances the body’s natural defenses against cancer by:
- Activation and Expansion of Immune Cells: Harvesting and stimulating the patient’s T-cells and NK cells to increase their numbers and activity.
- Targeted Attack on Cancer Cells: Reinforced immune cells are better equipped to recognize and destroy cancer cells.
- Overcoming Immune Suppression: Enhanced immune cells can counteract the tumor’s immunosuppressive strategies.
Clinical Evidence of Effectiveness
Clinical studies have demonstrated that Autologous Immune Cell Therapy can:
- Reduce Tumor Size: Significant shrinkage of tumors in various cancer types.
- Improve Survival Rates: Extended survival in patients with advanced cancers.
- Enhance Quality of Life: Fewer side effects compared to conventional therapies.
Types of Cancer Treated with Our Immune Cell Therapy
Our Japanese Autologous Immune Cell Therapy has shown promise in treating a variety of cancers, including 8 types of below cancers and many others:
Lung Cancer

Immune cell therapy reduces tumour size by up to 30%, significantly improving 5-year survival rates
Breast Cancer
Enhanced immune responses decrease recurrence by 25%, with tumour regression in over 60% of treated cases
Colorectal Cancer
Our therapy improves progression-free survival by 40%, targeting metastatic tumours with a 70% immune activation rate
Ovarian Cancer
Treatment enhances immune efficacy by 50%, achieving measurable tumour reduction in 65% of patients
Pancreatic Cancer
Immune cell therapy extends survival in advanced stages by 20%, improving tumour control rates by 35%
Prostate Cancer

Our therapy reduces PSA levels by 50% and delays progression in 70% of treated patients
Liver Cancer
Immune cell activation improves tumour response rates by 45%, with a 30% reduction in recurrence risk
Gastric Cancer

Our therapy increases overall survival by 15% and reduces tumour burden in over 50% of cases
Lung Cancer

Immune cell therapy reduces tumour size by up to 30%, significantly improving 5-year survival rates
Breast Cancer

Enhanced immune responses decrease recurrence by 25%, with tumour regression in over 60% of treated cases
Colorectal Cancer

Our therapy improves progression-free survival by 40%, targeting metastatic tumours with a 70% immune activation rate
Ovarian Cancer

Treatment enhances immune efficacy by 50%, achieving measurable tumour reduction in 65% of patients
Pancreatic Cancer

Immune cell therapy extends survival in advanced stages by 20%, improving tumour control rates by 35%
Prostate Cancer

Our therapy reduces PSA levels by 50% and delays progression in 70% of treated patients
Liver Cancer

Immune cell activation improves tumour response rates by 45%, with a 30% reduction in recurrence risk
Gastric Cancer

Our therapy increases overall survival by 15% and reduces tumour burden in over 50% of cases
…and many other cancers.
List of Treatments in MITAKY's Partners
MITAKY Group facilitates the following types of immune cell therapies through our official and certified partners’ medical centres and institutions:
| Therapy Type | Function | Benefits | Purpose |
|---|---|---|---|
| CST Cell Therapy | Activates cytotoxic T-cells to target and destroy cancer cells. | Enhances precision in attacking cancer cells, minimises damage to healthy tissues. | Cancers prevention. Effective against solid tumours, supports immune recovery in cancer patients. |
| CST + γδNKT Cell Therapy | Combines cytotoxic T-cells and γδNKT cells for broader immune activity. | Synergistic effects improve immune response and reduce tumour evasion mechanisms. | Cancers prevention / Cancers treatment. Treats a variety of cancers, including metastatic cancers. |
| CANK Cell Therapy (NK Cells) | Utilises Natural Killer (NK) cells to eliminate cancer through innate immune responses. | Boosts natural immune defence, reduces cancer recurrence rates. | Cancers prevention / Cancers treatment. Effective for residual cancer cells and advanced solid tumours. |
| Dendritic Cell Peptide Vaccine | Presents tumour-specific antigens to train the immune system to attack cancer cells. | Trains the immune system for long-term cancer surveillance and prevention of recurrence. | Cancers prevention / Cancers treatment. Supports personalised vaccines for advanced or recurrent cancers. |
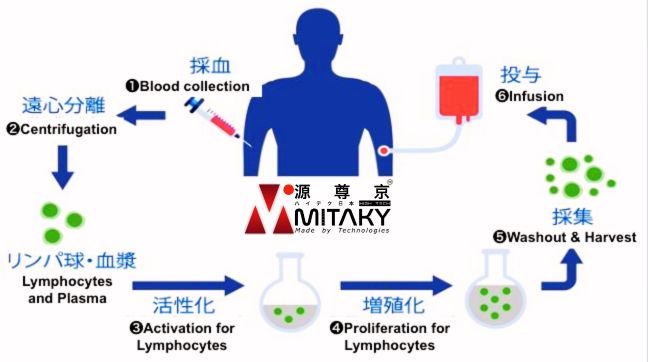
Process of Immune Cell Therapy
MITAKY Group ensures a seamless experience for international patients seeking Immune Cell therapy through our official partners of medical centres and institutions across Japan. The treatment process includes:
Consultations
Patients undergo a thorough medical evaluation to determine suitability for immune cell therapy. This includes reviewing medical history, current health status, and previous treatments.
✉️ Send your request to email: info@mitaky.co.jp
☎️ Hotline: +81-50-6875-7799
Blood Collection
A sample of the patient’s blood is collected, from which immune cells are isolated.
Cell Cultivation & Activation
The extracted immune cells are cultured and activated in a certified and controlled Cell Processing Centre (CPC) to enhance their cancer-fighting capabilities.
The cultured cells undergo strict quality checks to ensure safety, purity, and efficacy, adhering to Good Manufacturing Practice (GMP) standards.
What is CPC?
A Cell Processing Centre (CPC, 細胞培養加工施設) is a dedicated clean room that maintains the necessary cleanliness required for culturing cells. It is a specialised facility designed for handling and processing biological materials, such as stem cells, under stringent conditions. These facilities comply with the Ministry of Health, Labour and Welfare’s “Act on the Safety of Regenerative Medicine” (ASRM) and the “Pharmaceuticals and Medical Devices Act” (PMD Act). Additionally, to operate a CPC, the facility must obtain a specific facility number issued by the Minister of Health, Labour and Welfare in accordance with the ASRM. This ensures that all procedures meet Japan’s stringent safety and regulatory standards. CPCs operate under Good Manufacturing Practice (GMP) guidelines, which are internationally recognised standards in medical therapy production. Our partner centres follow these strict protocols to prevent contamination and ensure that the cells are of the highest quality.
Reinfusion
The enhanced immune cells are reinfused into the patient’s body via intravenous infusion, aiming to bolster the immune system’s ability to target and eliminate cancer cells.
Monitoring & Follow-Up
Post-treatment, patients are closely monitored to assess the therapy’s effectiveness and manage any potential side effects. Regular follow-up appointments at 1, 3, 6, and 12 months post-infusion are scheduled to evaluate progress and determine if additional treatment cycles are necessary.
Why Choose MITAKY Group for Your Immune Cell Therapy?
Access to Advanced
Technology
Our Japanese partner medical centres & institutes utilise the latest cell processing techniques, ensuring highly effective treatments with over 95% cell viability. Using own cells to maximise safety and effectiveness.
Absolute
Confidentiality
MITAKY Group is deeply committed to protecting the privacy and confidentiality of our patients, especially those who are affluent or high-profile individuals from businessmen and businesswomen to celebrities.
Personalised
Care
Treatments are tailored to each patient’s unique needs. With comprehensive supports in Japan: visa assistance, transportation, medical documents translation, professional interpretation, luxury shopping, resorts and golf clubs information.
Scientific
Validation
Our methods are supported by extensive research and clinical trials, ensuring scientifically validated treatments for conditions such as stroke, dementia, arteriosclerosis, knee pain, anti ageing and others.
Global
Standards
Our protocols meet international guidelines, offering treatments that are both safe and effective, recognised by health authorities worldwide.
Certified
Facilities
All stem cell processing and cultivation occur in CPC facilities that adhere to GMP standards, ensuring the highest quality and safety.
Japanese Excellence in Immune Cell Research
Japan has emerged as a global leader in immune cell research, driven by groundbreaking work at Kyoto University’s Center for iPS Cell Research and Application (CiRA), under Nobel Laureate Shinya Yamanaka. CiRA has pioneered the development of induced pluripotent stem (iPS) cell-derived immune cells, such as T cells and Natural Killer (NK) cells, designed to target and destroy cancer cells with precision. These advancements represent a significant step forward in cancer immunotherapy, offering new hope for patients through highly targeted and personalized treatments (CiRA Kyoto University).
Collaborative initiatives, such as the T-CiRA program with Takeda Pharmaceutical, aim to accelerate these innovations by creating off-the-shelf CAR T-cell therapies. These scalable and cost-effective treatments could make advanced immunotherapies more accessible globally. Researchers are also exploring methods to generate immune cells with reduced immunogenicity, enabling universal application from a limited number of iPS cells (T-CiRA Program). This integration of cutting-edge research and practical clinical development underscores Japan’s position as a leader in transformative cancer therapies.
Treatment Costs
The costs for Japanese Autologous Immune Cell Therapy facilitated through MITAKY Group’s partner medical centres are as follows:
- Initial Consultation and Testing: Starting from ¥110,000 (~USD 733).
- Cell Culturing Fees: ¥610,000–¥910,000 (~USD 4,148–6,460), depending on the type of therapy (e.g., NK, NKT, CST).
- Additional Fees: May apply for specialised tests or procedures.
Reference Exchange Rate: 1 JPY = 0.0068 USD.
Note: Prices include a 10% consumption tax. Final costs depend on individual treatment plans and specific medical needs. For more details, please contact MITAKY Group directly.
Our Medical Partners' Facilities
The immune cells are prepared at our own on-site certified cell culture and processing facility (CPC), for the therapy.
We also store the cells under our reliable management system that complies with national and legal standards, specially to our Japan’s Ministry of Health, Labor and Welfare.





Frequently Asked Questions
Immune cell therapy is a treatment that extracts, activates, and reinfuses a patient’s immune cells to strengthen their ability to recognize and destroy cancer cells.
Yes, immune cell therapy is generally safe because it uses the patient’s own immune cells, which eliminates the risk of rejection or severe allergic reactions. The procedures involved, such as blood collection and reinfusion, are minimally invasive. Additionally, immune cells are processed in controlled, high-standard facilities that adhere to strict safety and quality regulations, such as GMP (Good Manufacturing Practice). Compared to conventional treatments like chemotherapy or radiation, which can damage healthy tissues, immune cell therapy targets cancer cells specifically, resulting in fewer and milder side effects, such as temporary fatigue or slight fever. Severe complications are rare.
The therapy boosts the immune system by activating and expanding immune cells like T cells and NK cells, which target and destroy cancer cells more effectively.
This therapy is effective for cancers such as lung, breast, colorectal, ovarian, pancreatic, liver, gastric, prostate cancers, and rare cancers like thymus and bone cancer.
Side effects are typically rare and may include fever, fatigue, or temporary inflammation at the injection site.
Unlike chemotherapy or radiation, immune cell therapy enhances the body’s natural defenses, minimising damage to healthy cells and reducing side effects.
Yes, it complements surgery, chemotherapy, or radiotherapy, enhancing overall treatment effectiveness and improving quality of life.
The process typically involves 2–3 weeks for immune cell activation, with multiple reinfusion sessions spanning several months depending on the treatment plan.
No, immune cell therapy is performed on an outpatient basis, allowing patients to resume their daily activities on the same day.
Effectiveness varies based on cancer type, stage, and individual factors. Many patients experience reduced tumour size, prolonged survival, and improved quality of life.
For more about testimonials of our past patients, please contact us for more details.
Yes, it strengthens the immune system to target residual cancer cells, reducing the risk of recurrence and improving long-term outcomes.
No, it is classified as advanced medical care and is not covered by public health insurance in Japan.
Some private insurance plans may provide partial coverage. It is advisable to check with your insurer for details about eligibility and reimbursement.
Costs vary but typically include consultation fees, cell activation, and reinfusion charges. Contact us for a personalised estimate.
Patients with early-stage, advanced, or recurrent cancers may be eligible. Preventative therapy may also benefit high-risk individuals.
There are generally no strict age restrictions. Eligibility depends on the patient’s overall health and cancer stage as determined during consultation.
No, the procedures, including blood collection and reinfusion, are minimally invasive and cause little discomfort.
The therapy reduces side effects, boosts energy levels, and strengthens the immune system, improving overall well-being.
Yes, it is often recommended for patients who have not responded to chemotherapy or radiation, providing an alternative approach.
Yes, MITAKY Group specialises in assisting international patients by connecting them with top medical centres in Japan. We provide comprehensive support, including medical arrangements, language interpretation, and travel logistics, ensuring a seamless experience.
Begin by scheduling a consultation. A medical specialist will assess your condition and develop a personalised treatment plan.
MITAKY Group is committed to providing international patients with access to Japan’s highest quality medical treatments through our network of accredited medical centres & institutes. By choosing MITAKY, you benefit from world-class care, cutting-edge technology, and personalised treatment plans that leverage the latest advancements in regenerative medicine. Let us connect you to a healthier, brighter future.
For more information or to schedule a consultation, please contact our support team by an official enquiry form below or by:
- Email at info@mitaky.co.jp (Takahashi, and Tamura)
- Hotline: +81-50-6875-7799



